Abstract

Telomere biology disorders (TBD) are caused by germline mutations in telomere related genes that lead to excessive telomere shortening. Affected genes include TERT and TERC, which encode two components of the telomerase complex. TBDs are multisystem disorders that present with a constellation of findings including bone marrow failure and lung and/or liver disease. Immunodeficiency has been described but is not well characterized in a large cohort of TBD patients. In the present study, we report the clinical incidence and severity of infections, as well as laboratory evidence of immunodeficiency in 83 patients with TBD.
The electronic medical record was queried for the clinical history of patients with TBD who were evaluated at the National Institutes of Health Clinical Center from 2002 to present. Laboratory data included immunoglobulins (IgG, IgA, IgM) and peripheral blood lymphocyte subsets (CD4, CD19). Fifteen patients did not have immunoglobulin levels and 29 patients did not have lymphocyte subsets available. A significant infection history was defined as occurrence of infections that were opportunistic, recurrent, and/or required hospitalization. Recurrent infections were defined as two or more severe infections in one year, three or more respiratory infections (e.g. sinusitis, otitis, bronchitis) in one year, or the need for antibiotics for two months per year. Diminished immune function was defined by CD4 T cell counts: patients with CD4 < 200 cells x 106/L were classified as immunodeficient, and CD4 >200 cells x 106/L but < 500 cells x 106/L as immune impaired. Reference ranges for female and male patients were used to determine whether levels are below or within normal limits. Development of lung and/or liver fibrosis were also assessed. Chi-square tests were used to test the association between the two categorical variables. Kaplan-Meier curves and log rank tests were used to estimate overall survival (OS) and test the differences of survival time distributions among infection groups and immune status groups.
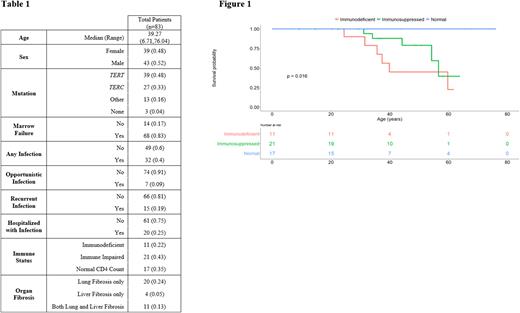
Patient characteristics are in Table 1. TERT and TERC were the most commonly mutated genes, and others included DKC1, PARN, TINF2, CTC1, RTEL1, and NAF1. Of 49 patients with laboratory data available, the majority (65%) had depressed CD4 counts with 11 immunodeficient patients, 21 immune impaired patients, and 17 patients with normal counts. Median age was similar in the 3 groups. Three (27%) immunodeficient patients and 2 (10%) immune impaired patients had an opportunistic infection. Two (18%) immunodeficient patients and 6 (29%) immune impaired patients reported recurrent infections. Nine (43%) immune impaired patients required hospitalization from infection. No patients with normal CD4 count had opportunistic or recurrent infections, but 4 had required hospitalization for infection. Lower CD4 count was associated with having a significant infection history, and specifically recurrent infections, and being hospitalized for infection (p=0.0417, p=0.0471, p=0.0154 respectively). We observed varied associations between other clinical factors and immune dysfunction or infection. Low levels of CD19 B cells were observed in 14 patients, and associated with development of recurrent infections (P = 0.0003) but not opportunistic or infections requiring hospitalization. Two patients had deficient IgG and IgA levels while 8 had deficient IgM. However, immunoglobulins were not associated with any significant infection history. All immunodeficient and immune impaired patients had concurrent bone marrow failure. Lung fibrosis was recorded in 6 (54%) immunodeficient and 6 (27%) immune impaired patients, and liver fibrosis in 4 (36%) immunodeficient and 4 (19%) immune impaired patients. Of 11 patients with both lung and liver fibrosis, 4 (36%) were immunodeficient, 2 (18%) were immune impaired, and 5 (45%) did not have CD4 counts available. There was no association between gene mutation and development of low CD4 count or clinically significant infections. OS was lower in TBD patients with clinically significant infections (p=0.00011) or low CD4 counts (p=0.016) (Figure 1).
Immune dysfunction is common in TBD and patients are at increased risk of clinically significant infections. Low CD4 counts are associated with worse OS. TBD patients should be routinely screened for immunodeficiency to allow appropriate clinical monitoring, antimicrobial prophylaxis, and education.
Disclosures
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal